
Hong Ji, MD
Biosketch
Associate Professor
Department of Medicine
Georgetown University Medical Center
Cloning of angiotensin receptors
After four years of postdoctoral training at NIH via an NICHD Intramural Research Training Award and through the NIGMS Pharmacology Research Associate Program, I accepted a Senior Staff Fellow position in the NICHD in 1991 and engaged in a project to clone the angiotensin receptor. The first step was to develop an expression assay using Xenopus oocytes. This project led to the discovery that the follicular cells surrounding oocytes express angiotensin II (Ang II) receptors that upon agonist stimulation induce calcium mobilization in the oocyte by signaling through gap junctions ( Sandberg, et al., Science 1990; Sandberg et al., JCB, 1992)). This research also led to the observation that mammalian and amphibian Ang II receptors bind peptide agonists with similar affinities but display distinct pharmacological differences in nonpeptide ligand affinities ( Sandberg et al., FEBS Lett, 1991). These early studies lay the way to our successful cloning of the rat angiotensin type 1b receptor (AT1bR) (Sandberg et al., JBC, 1992) and subsequently, the Xenopus type a angiotensin receptor (xATaR).
After four years of postdoctoral training at NIH via an NICHD Intramural Research Training Award and through the NIGMS Pharmacology Research Associate Program, I accepted a Senior Staff Fellow position in the NICHD in 1991 and engaged in a project to clone the angiotensin receptor. The first step was to develop an expression assay using Xenopus oocytes. This project led to the discovery that the follicular cells surrounding oocytes express angiotensin II (Ang II) receptors that upon agonist stimulation induce calcium mobilization in the oocyte by signaling through gap junctions ( Sandberg, et al., Science 1990; Sandberg et al., JCB, 1992)). This research also led to the observation that mammalian and amphibian Ang II receptors bind peptide agonists with similar affinities but display distinct pharmacological differences in nonpeptide ligand affinities ( Sandberg et al., FEBS Lett, 1991). These early studies lay the way to our successful cloning of the rat angiotensin type 1b receptor (AT1bR) (Sandberg et al., JBC, 1992) and subsequently, the Xenopus type a angiotensin receptor (xATaR).
After cloning the AT1bR and xATaRs, I began studying structure function determinants of ligand binding and signaling. This led to the
identification of amino acids that were critical for binding a then newly discovered class of AT1R nonpeptide antagonists
(Sandberg et al. JBC, 1994)
and that are now widely used clinically to treat hypertension and cardio-renal diseases. After I accepted
a tenure-track faculty position as Assistant Professor at GU, in addition to continuing my structure function research
(Ji et al., PNAS, 1995),
I began investigating the regulation of the AT1R and found that 17β-estradiol regulates Ang II-induced aldosterone secretion by modulating
plasma Ang II
(Roesch et al., Endocrinology, 2000;
Wu et al., Endocrinology, 2003).
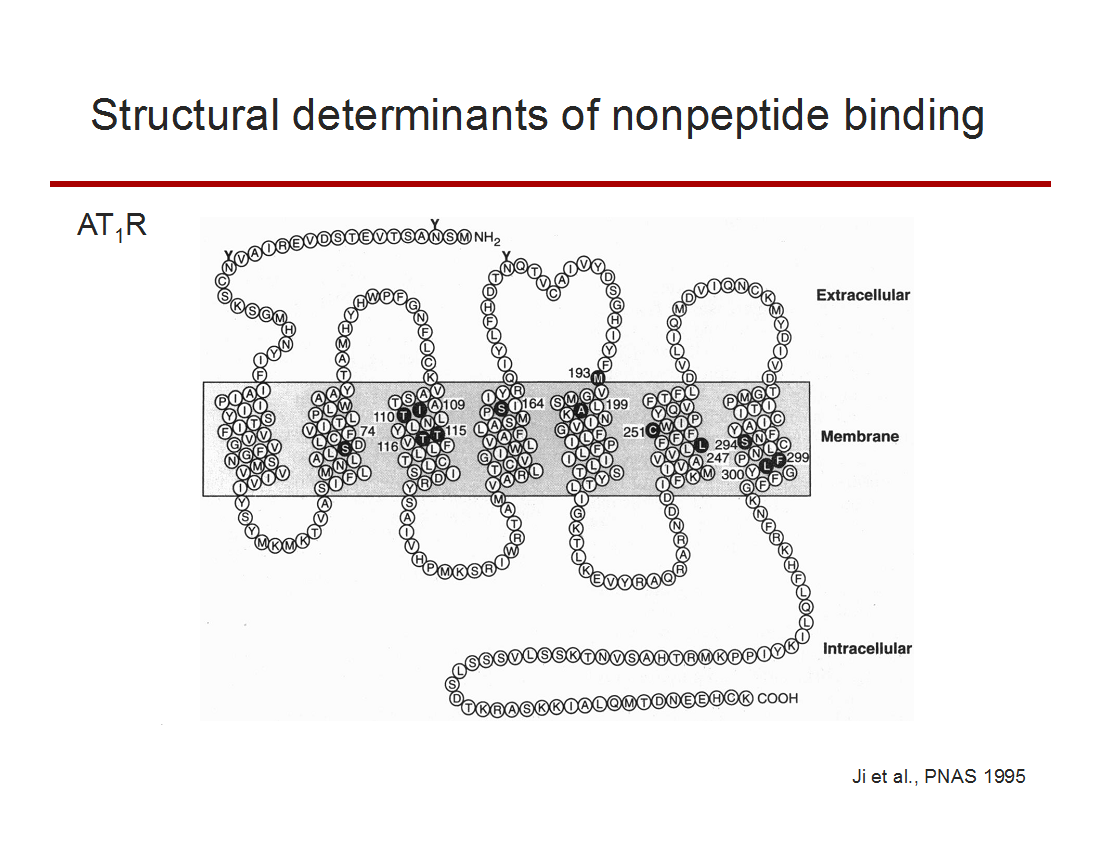
Schematic representation of the rat angiotensin type 1 receptor (AT1R). Solid circles with white type indicate the nonconserved amino acids in the amphibian angiotensin type a receptor that correspond to the mammalian residues influential in Losartan binding to the AT1R.
Posttranscriptional regulation of the angiotensin receptors
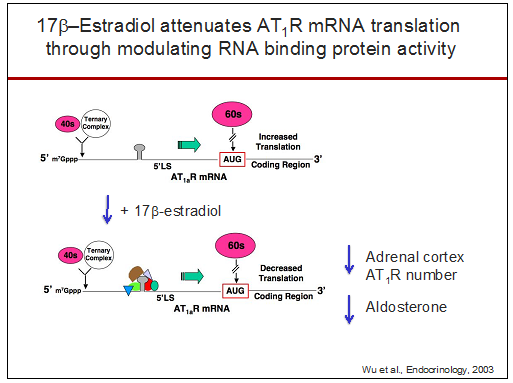
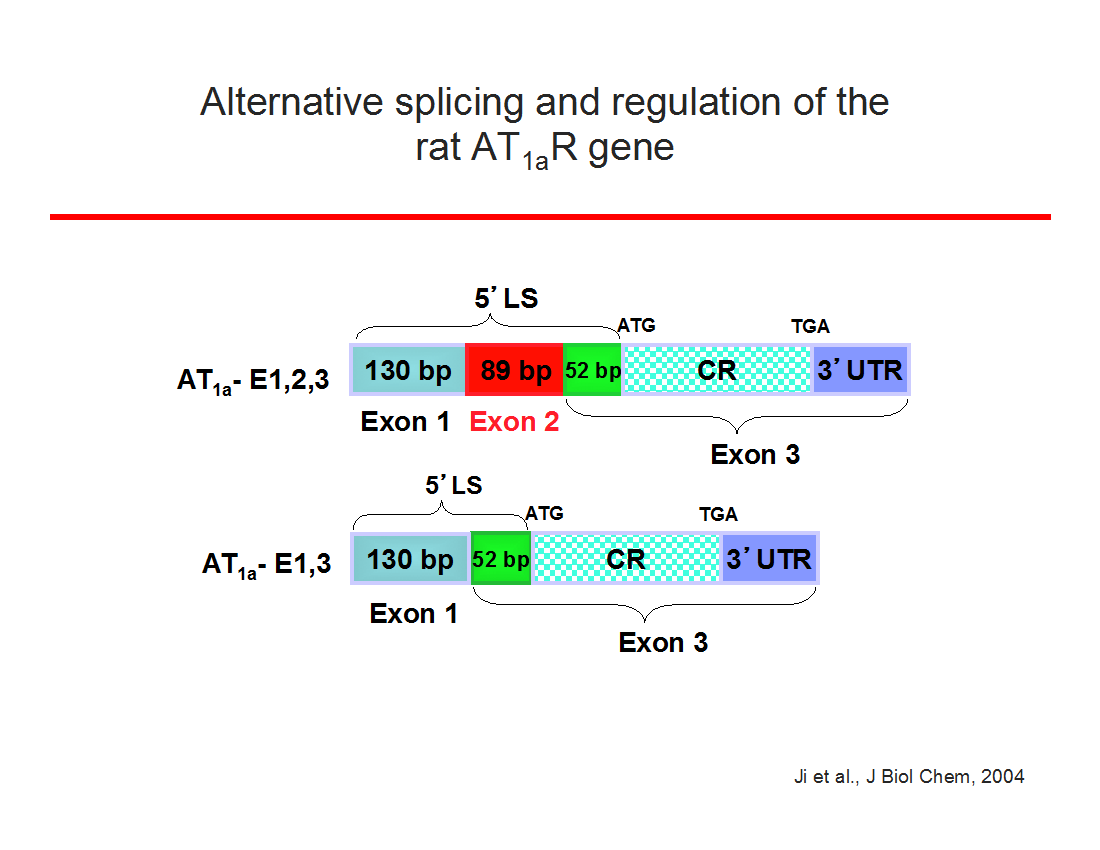
During my laboratory's research on 17β-estradiol regulation of AT1Rs in the adrenal, we discovered that RNA binding proteins regulate receptor function by selectively binding within exon 2 of the AT1aR 5'-leader sequence (Wu et al., Endocrinology, 2003) and that this translational regulation is mediated by a short open reading frame in exon 2 ( Ji et al, JBC 2004). Furthermore, we found this new mechanism of posttranscriptional regulation is shared with other peptide hormone G protein-coupled receptors ( Wu et al., Neuroendocrinology, 2004 ). More recently, this research direction has led to the exciting discovery that a seven amino acid peptide (PEP7) encoded with a short open reading frame within exon 2 is a selective inhibitor of AT1aR signaling; PEP7 inhibits the Erk1/2 but not the classic inositol trisphosphate pathway ( Liu et al., AJP: Reg, 2014).
During my laboratory's research on 17β-estradiol regulation of AT1Rs in the adrenal, we discovered that RNA binding proteins regulate receptor function by selectively binding within exon 2 of the AT1aR 5'-leader sequence (Wu et al., Endocrinology, 2003) and that this translational regulation is mediated by a short open reading frame in exon 2 ( Ji et al, JBC 2004). Furthermore, we found this new mechanism of posttranscriptional regulation is shared with other peptide hormone G protein-coupled receptors ( Wu et al., Neuroendocrinology, 2004 ). More recently, this research direction has led to the exciting discovery that a seven amino acid peptide (PEP7) encoded with a short open reading frame within exon 2 is a selective inhibitor of AT1aR signaling; PEP7 inhibits the Erk1/2 but not the classic inositol trisphosphate pathway ( Liu et al., AJP: Reg, 2014).
Impact of gonadal hormones and sex chromosomes in renovascular disease.
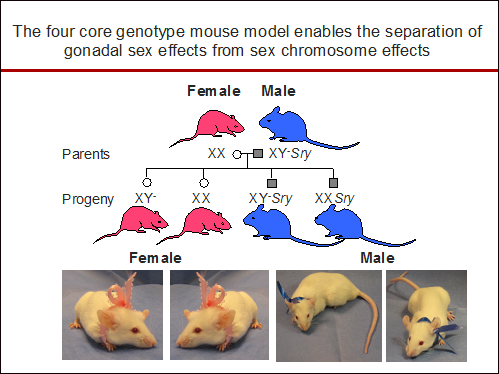
Our research on 17β-estradiol regulation of AT1R function led to studies investigating why females are protected from hypertension and chronic kidney disease. We found ovariectomy augments the magnitude of hypertension in aging salt-sensitive rats ( Hinojosa-Laborde et al., Hypertension, 2004 ) as well as the progression of renal injury in a model of renal-induced hypertension ( Ji et al., AJP: Heart, 2005 ). In addition, we found 17β-estradiol protected against the progression of hypertension and renal disease in part by down-regulating the expression of AT1Rs ( Hinojosa-Laborde et al., Hypertension, 2004; Ji et al., AJP: Renal, 2005 ). Using the unique four core genotype mouse animal model in which gonadal sex is separated from the sex chromosomes, we found the sex chromosome complement impacts the magnitude of the hypertension independently of gonadal hormones ( Ji et al., Hypertension 2010 ), which was the report of a sex chromosome effect in hypertension.
Our research on 17β-estradiol regulation of AT1R function led to studies investigating why females are protected from hypertension and chronic kidney disease. We found ovariectomy augments the magnitude of hypertension in aging salt-sensitive rats ( Hinojosa-Laborde et al., Hypertension, 2004 ) as well as the progression of renal injury in a model of renal-induced hypertension ( Ji et al., AJP: Heart, 2005 ). In addition, we found 17β-estradiol protected against the progression of hypertension and renal disease in part by down-regulating the expression of AT1Rs ( Hinojosa-Laborde et al., Hypertension, 2004; Ji et al., AJP: Renal, 2005 ). Using the unique four core genotype mouse animal model in which gonadal sex is separated from the sex chromosomes, we found the sex chromosome complement impacts the magnitude of the hypertension independently of gonadal hormones ( Ji et al., Hypertension 2010 ), which was the report of a sex chromosome effect in hypertension.
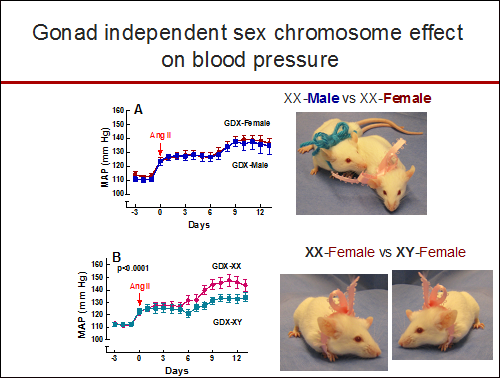
Regardless of being born male or female, the mean arterial pressure (MAP) response after angiotensin II (Ang II) infusion was greater in gonadectomized mice with the XX sex chromosome complement (GDX-XX) compared to gonadectomized mice with the XY sex chromosome complement (GDX-XY).
Sex-specific immune modulation of hypertension
Our most recent studies focus on the role of biological sex in immune modulation of hypertension. We have found that male T cells exacerbate Ang II-induced hypertension in the male mouse but are unable to drive hypertension in the female ( Pollow et al., Hypertension, 2014 ). Furthermore, female T cells are not able to drive the hypertension in the male mouse ( Ji et al., Hypertension, 2014; Sandberg et al., Cell Immunology, 2015 ). These findings indicate biological sex is a critical determinant in T cell differentiation and/or function and emphasize the importance of the recent NIH initiative to balance the sex of cells and animals in preclinical studies.
Our most recent studies focus on the role of biological sex in immune modulation of hypertension. We have found that male T cells exacerbate Ang II-induced hypertension in the male mouse but are unable to drive hypertension in the female ( Pollow et al., Hypertension, 2014 ). Furthermore, female T cells are not able to drive the hypertension in the male mouse ( Ji et al., Hypertension, 2014; Sandberg et al., Cell Immunology, 2015 ). These findings indicate biological sex is a critical determinant in T cell differentiation and/or function and emphasize the importance of the recent NIH initiative to balance the sex of cells and animals in preclinical studies.
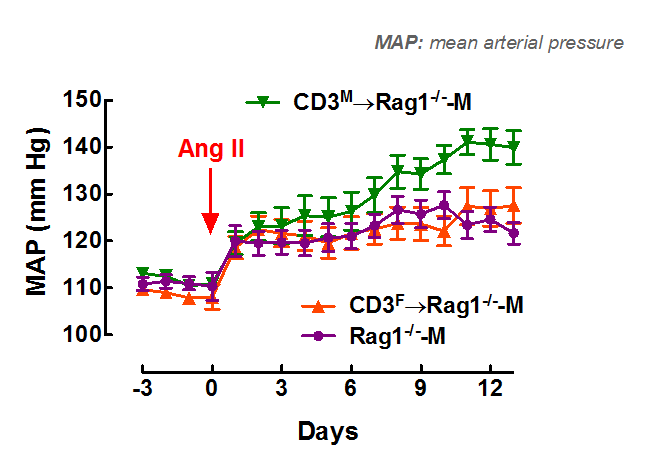
Adoptive transfer of male T cells (CD3M) into male recombination-activating- gene-1- deficient mice (Rag1-/--M) – mice deficient in T cells – (CD3M→Rag1-/--M) resulted in a greater increase in mean arterial pressure (MAP) in response to angiotensin II (Ang II) compared to adoptive transfer of female T cells (CD3F→Rag1-/--M).
